2. Development of Small Boron-containing Molecules as BNCT Agents
Author: Martin Kellert and Evamarie Hey-Hawkins
© Dr. Christoph Selg
2.1. Amino Acid Derivatives
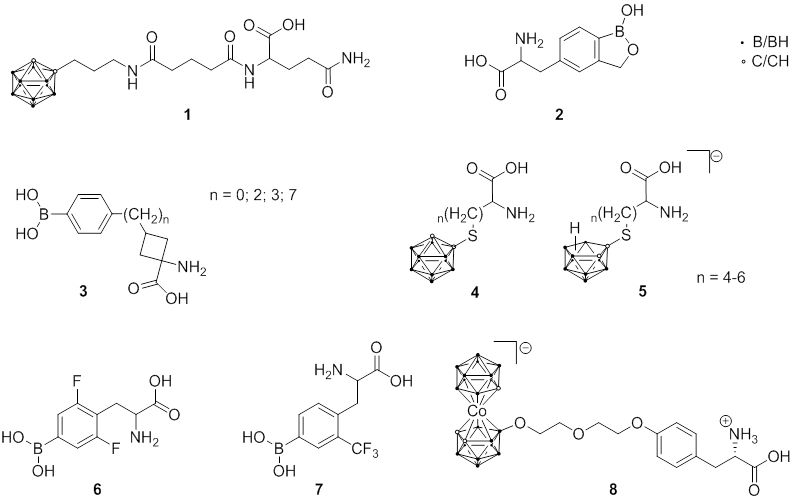
Fig. 2.1 Structures of boron-containing amino acids
2.2. Nucleic Acid Derivatives
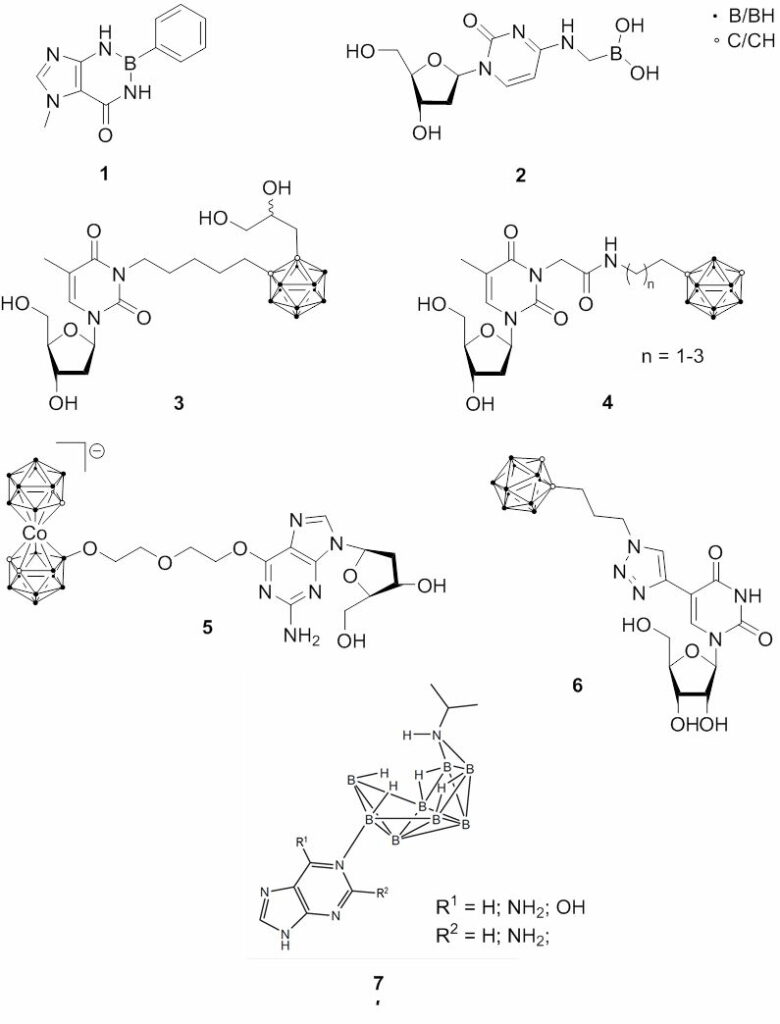
Fig. 2.2 Structures of boron-containing nucleosides
2.3. Boronated Porphyrins and Their Analogs
Porphyrins and their analogs such as chlorins , phthalocyanines and porphyrazines are known to accumulate selectively in a wide variety of tumors, and these compounds are used in photodynamic therapy (PDT) for cancer treatment as photosensitizers .
Boronated porphyrins are used in both BNCT and PDT, and as theranostic drugs because their distribution in cells and tissues can be visualized easily by fluorescence imaging .
The boronated porphyrin BOPP (1) was selectively taken up by tumor cells in xenograft models of glioma and localized predominantly in the mitochondria . A phase I drug evaluation of BOPP was subsequently carried out. However, BOPP could not be used clinically because thrombocytopenia was observed in patients due to its toxic effect and/or that of its metabolites on the platelets.
Various other boronated porphyrins and their analogs have been synthesized and evaluated as boron carriers for BNCT to overcome these side effects. Most of the boronated porphyrins containing one or more boronic acid groups or carboranes in the porphyrin backbone have poor water solubility. Therefore, porphyrins with ionic boron clusters such as nido-carborate, dodecaborate, and cobalt bis(dicarbollide) were developed to increase the water solubility. A dodecaborate containing protoporphyrin (2) was developed, which showed high water solubility and low cytotoxicity, and exhibited higher intercellular boron concentrations in various tumor cells than BOPP. A chlorin derivative with two BPA moieties (3) as substituents showed good water solubility (100 mg/mL) and tumor selectivity.
Carborane-containing porphyrins were conjugated with linear and branched polyamine and opioid peptide (Tyr-D-Arg-Phe-β-Ala; YRFA) (4) to increase the bloodbrain barrier (BBB) permeability. These conjugates showed low cytotoxicity (> 400 μM) and a high BBB permeability coefficient in an in vitro model .
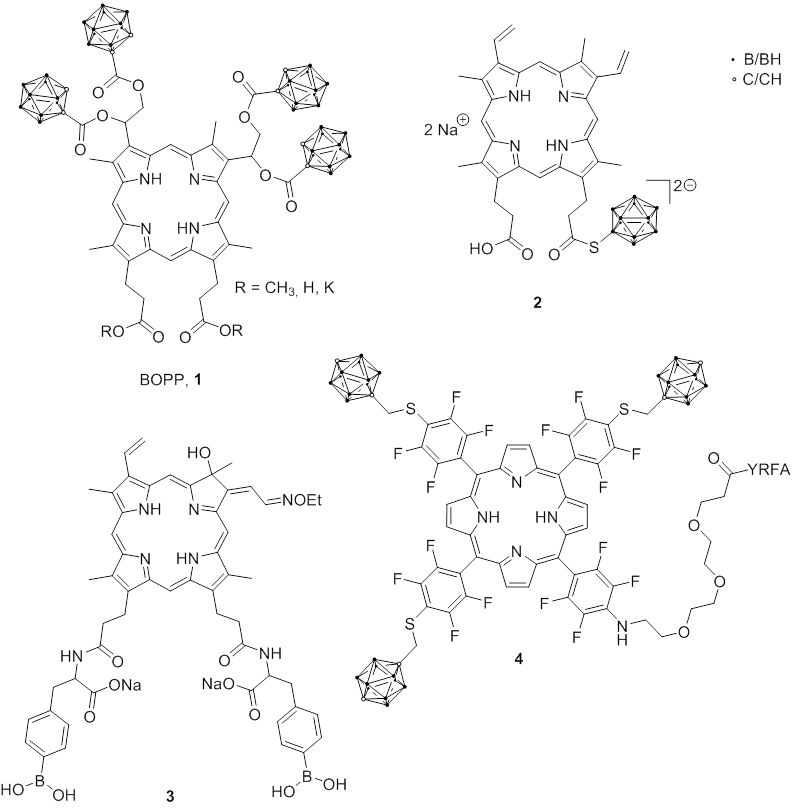
Fig. 2.3 Boronated Porphyrins and Chlorin
2.4. Carbohydrates
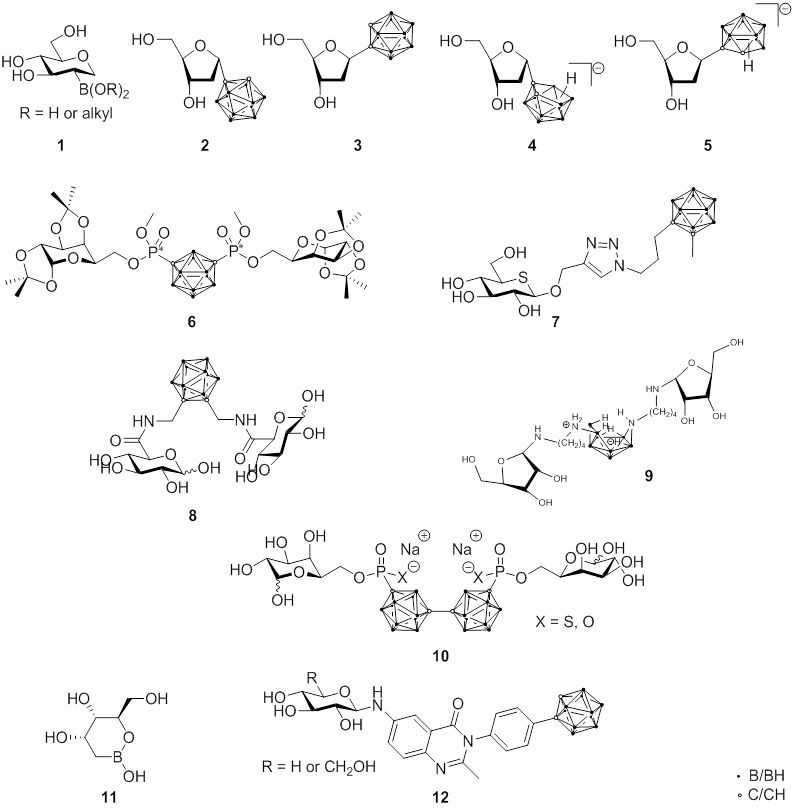
Fig. 2.4 Carbohydrate-containing boron compounds
2.5. Peptides
The groups of Hey-Hawkins and Beck-Sickinger have focused on different peptides which show selectivity towards G protein-coupled receptors which are overexpressed in different cancer types. Recent research included the synthesis of closo-carborane-modified neuropeptide Y analogs or metallacarborane derivatives (1); a second approach was the incorporation of meta-carboranes in novel ghrelin receptor agonists. More interesting is the improvement of these derivatives by introduction of water-soluble side chains at the carborane moieties (2), whereas the change from D-galactosyl to L-galactosyl groups increased the selectivity of these derivatives due to a lower unspecific uptake of bioconjugates into liver tissue.
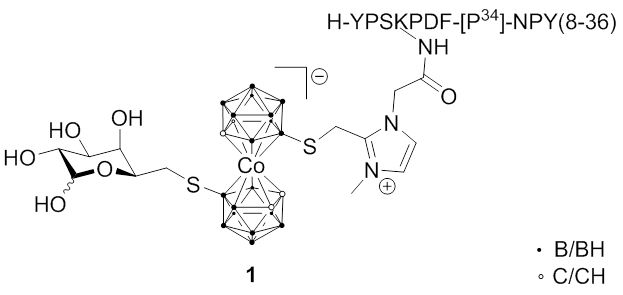
Fig. 2.5a Metallacarborane conjugate 1 with tumor-selective neuropeptide Y
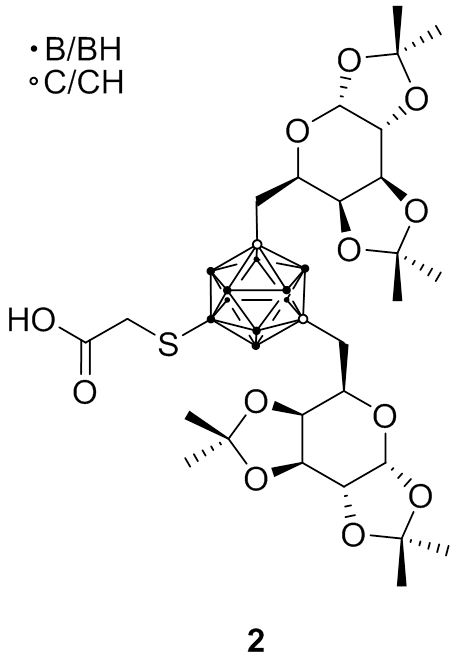
Fig. 2.5b Bis-D-galactosylated meta-carborane 2 as a water-soluble building block for tumor-selective peptides
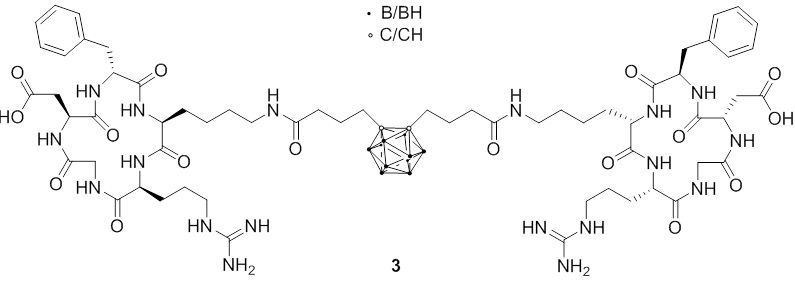
Fig. 2.5c cRGD-peptide conjugate with ortho-carborane
2.6. DNA- or Mitochondria-targeting Molecules
One of the important characteristics of boron carriers is the localization in the cytosol and/or nucleus of cancer cells. If boron compounds can be specifically accumulated in the nucleus, the DNA of cancer cells will be destroyed more effectively by BNCT. Based on this strategy, the accumulation of boron-containing compounds such as polyamine, DNA binding agents, and DNA intercalators have been reported. Within this approach, a carborane-containing-polyamine, ASPD-5 (1), was prepared and showed a high binding affinity for DNA and high tumor selectivity. Furthermore, in some cases it might be possible to combine BNCT properties with chemotherapeutic aspects to increase the overall therapeutic effect, for example, with boronated cisplatin -containing closo-carborane (2) or nido-carborate or carborane-containing benzo[b]acridones (DNA intercalator ) (3).
Another specific intracellular target are mitochondria . One strategy to address mitochondria as BNCT target is the combination of carboranes or dodecaborates with selected amino acid sequences which are known to be selective towards mitochondria; one example is the conjugation of BSH with an RLA (arginine-leucine-alanine) peptide (4).
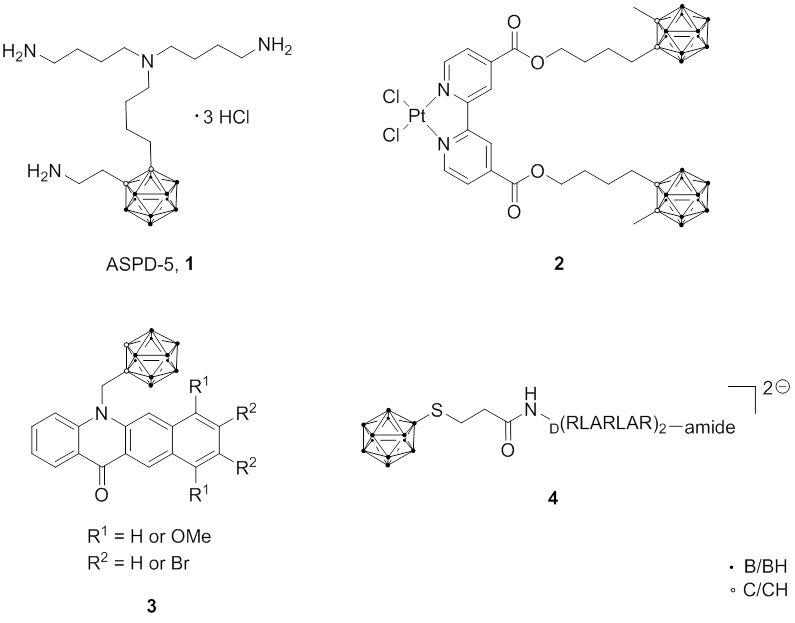
Fig. 2.6 Boron compounds for targeting DNA or mitochondria
1. Basic Requirements of BNCT – BSH and BPA
2. Development of Small Boron-containing Molecules as BNCT Agents
2.1. Amino Acid Derivatives
2.2. Nucleic Acid Derivatives
2.3. Boronated Porphyrins and Their Analogs
2.4. Carbohydrates
2.5. Peptides
2.6. DNA- or Mitochondria-targeting Molecules
3. Boron Compounds Conjugated with Biomolecules
3.1. Boron-conjugated Growth Factors
3.2. Boron-Conjugated Antibodies
3.3. Boron-conjugated Proteins
4. Boronated Polymers, Liposomes, and Other Nanoparticles as Boron-delivery Systems
4.1. Boronated Polymers and Micelles
4.2. Boronated Biopolymers
4.3. Emulsions
4.4. Liposomes
4.5. Boronated Nanoparticles
4.6. Boronated Nanotubes
