4. Boronated Polymers, Liposomes, and Other Nanoparticles as Boron-delivery Systems
Author: Martin Kellert and Evamarie Hey-Hawkins
© Dr. Christoph Selg
4.1. Boronated Polymers and Micelles
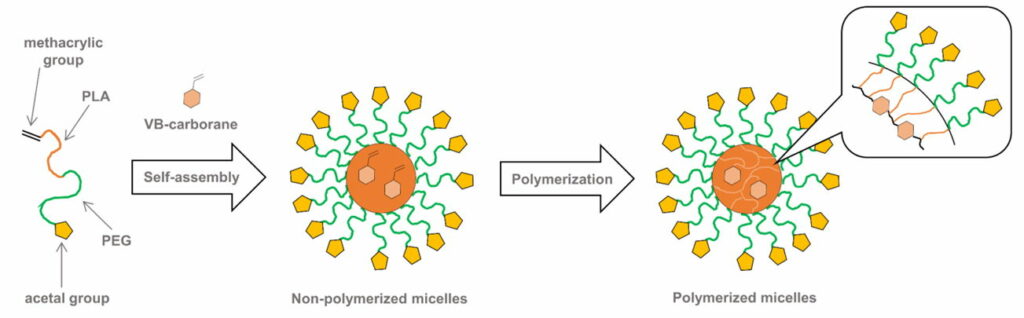
Fig. 4.1a Schematic illustration of the CL micelles containing PEG-b-PLA copolymer
Doxorubicin -loaded carborane-conjugated polymeric nanoparticles (DOX@PLMB) (Fig. 4.1b) were prepared to combine the treatment effects of BNCT and anticancer drug-based chemotherapy. Indeed, the DOX@PLMB nanoparticles selectively delivered boron atoms and doxorubicin to the tumor site of mice simultaneously and enhanced the tumor suppression efficiency compared to doxorubicin-loaded polymeric nanoparticles.
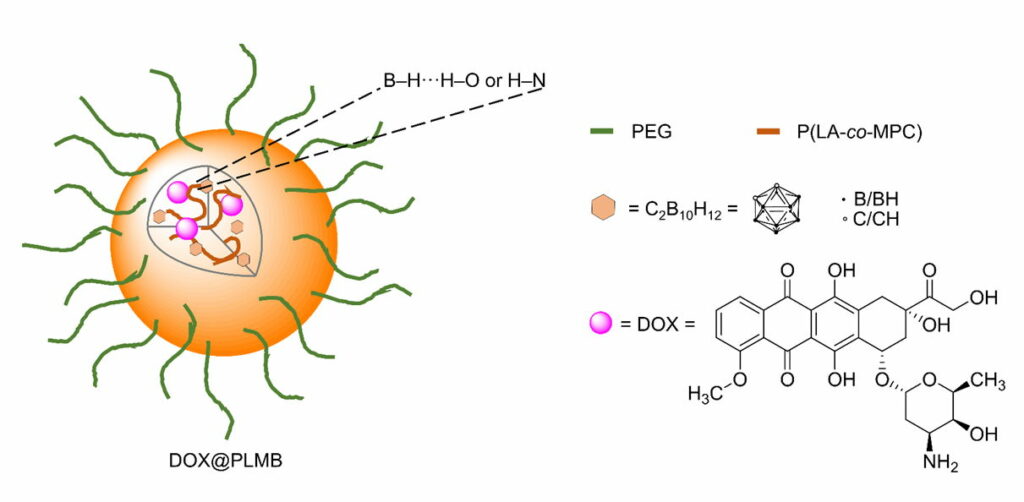
Fig. 4.1b Schematic illustration of DOX@PLMB with its components
4.2. Boronated Biopolymers
Hyaluronic acid (HA) is a glycosaminoglycan distributed widely throughout connective, epithelial, and neural tissues. As one of the chief components of the extracellular matrix, HA contributes significantly to cell proliferation and migration, and may also be involved in the progression of some malignant tumors. Therefore, HA has emerged as a promising candidate for intracellular delivery of various therapeutic and imaging agents due to its ability to recognize specific cellular receptors, mainly the CD44 antigen that is overexpressed in many cancer histotypes.
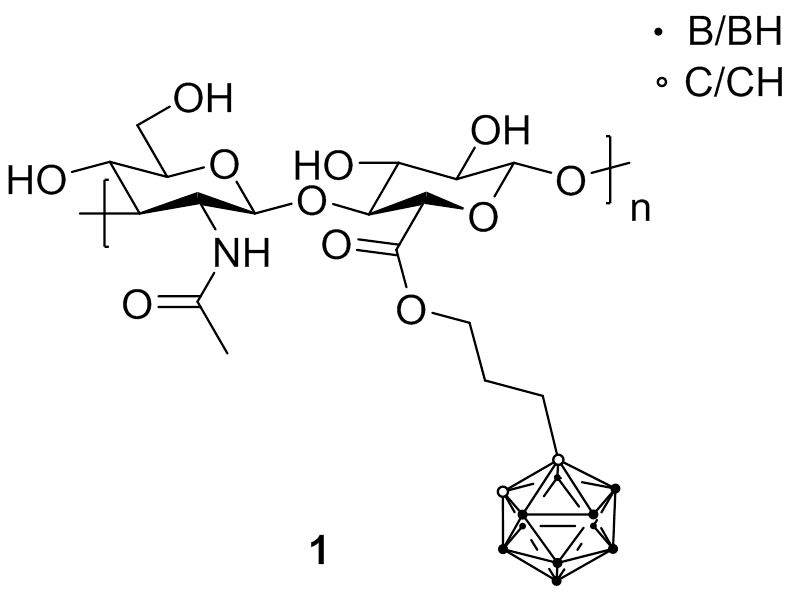
First attempts to combine boron sources with HA were conducted with carboranes (1). Biological studies revealed that the boronated HA retained the capacity to specifically interact with CD44 receptor and be internalized to achieve an elevated boron atom content within cells.
4.3. Emulsions
4.4. Liposomes
4.5. Boronated Nanoparticles
Silver and gold nanoparticles have recently received considerable interest as possible vehicles for drug delivery because of their peculiar physicochemical properties, such as surface plasmon resonance in the visible or near infrared region, photoluminescence , and tunable chemical functionalization through bonding with thiol, amine or carboxylic acid groups.
Water-soluble carborane-functionalized silver nanoparticles conjugated with anti-EGFR antibodies were developed. Surface-enhanced Raman scattering (SERS) microscopy revealed that the modified silver nanoparticles (Fig. 4.5) were selectively targeting an extracellular domain of the EGF receptor. It was estimated that a single 25 nm nanoparticle (surface area ~2000 nm2) with a 45% carborane coating would carry approximately 9000 carborane molecules per targeted antibody.
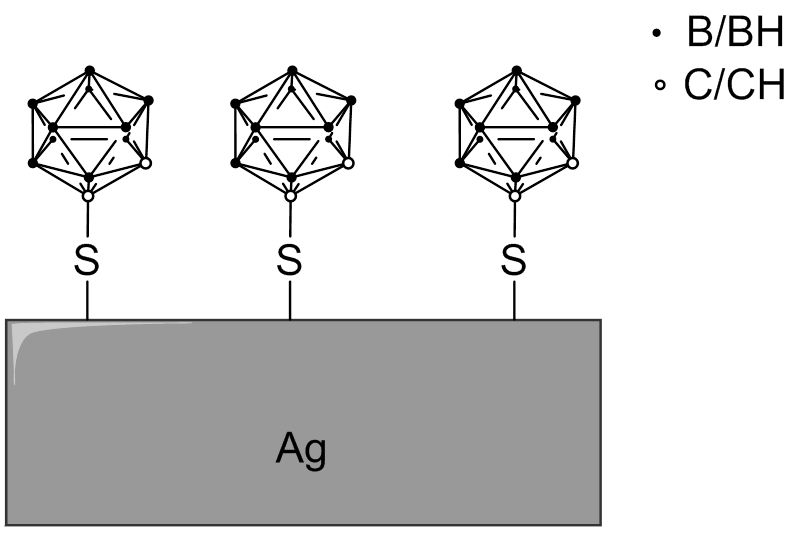
Fig. 4.5 Schematic surface of a silver nanoparticle modified with 1-mercapto-1,2-dicarba-closo-dodecaborane
Furthermore, also gold nanoparticles were applied as boron carriers. For instance, gold nanoparticles, multi-functionalized with BPA, folate, and fluorescent dye for imaging were prepared employing the layer-by-layer coating method. Confocal fluorescence microscopy demonstrated significant uptake of the functionalized gold nanoparticles in folate receptor -overexpressing cancer cells.
4.6. Boronated Nanotubes
As functionalized single-wall carbon nanotubes (SWCNTs) are able to cross cell membranes and concentrate in a number of neoplastic cells without obvious toxic effects, nido-carborates were attached to SWCNTs to produce water-soluble macromolecular boron carriers (Fig. 4.6a). In vivo studies with mice bearing an EMT6 mammary cancer tumor showed 21.5 B/g tumor and a tumor-to-blood ratio of 3.12 : 1.
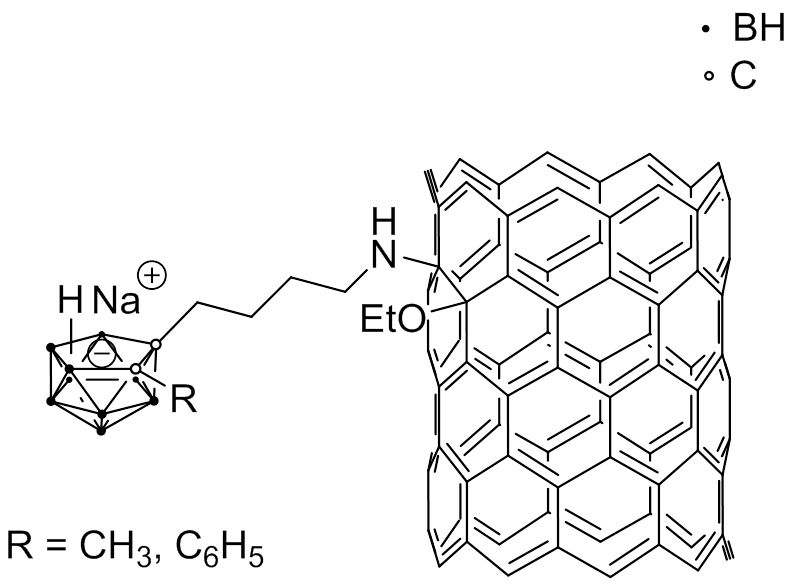
Fig. 4.6a Example of a SWCNT modified with nido-carborate
Boron nitride nanotubes (BNNTs) are analogs of CNTs, in which the carbon atoms are substituted by alternating boron and nitrogen atoms. It was shown that folate-conjugated BNNTs (Fig. 4.6b) exhibited an increased up-take by HeLa cells , probably because of receptor-mediated endocytosis, and co-localized with the lysosomes in the cells.
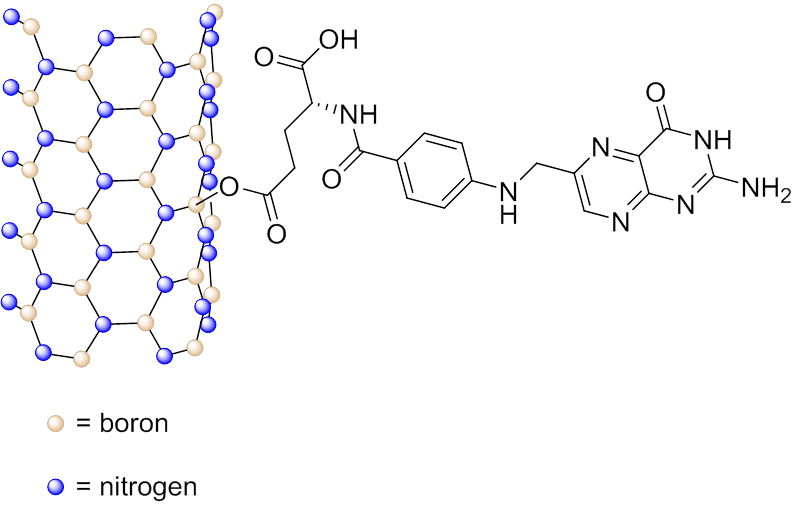
Fig. 4.6b Schematic representation of folic acid conjugated to a BNNT
1. Basic Requirements of BNCT – BSH and BPA
2. Development of Small Boron-containing Molecules as BNCT Agents
2.1. Amino Acid Derivatives
2.2. Nucleic Acid Derivatives
2.3. Boronated Porphyrins and Their Analogs
2.4. Carbohydrates
2.5. Peptides
2.6. DNA- or Mitochondria-targeting Molecules
3. Boron Compounds Conjugated with Biomolecules
3.1. Boron-conjugated Growth Factors
3.2. Boron-Conjugated Antibodies
3.3. Boron-conjugated Proteins
4. Boronated Polymers, Liposomes, and Other Nanoparticles as Boron-delivery Systems
4.1. Boronated Polymers and Micelles
4.2. Boronated Biopolymers
4.3. Emulsions
4.4. Liposomes
4.5. Boronated Nanoparticles
4.6. Boronated Nanotubes
